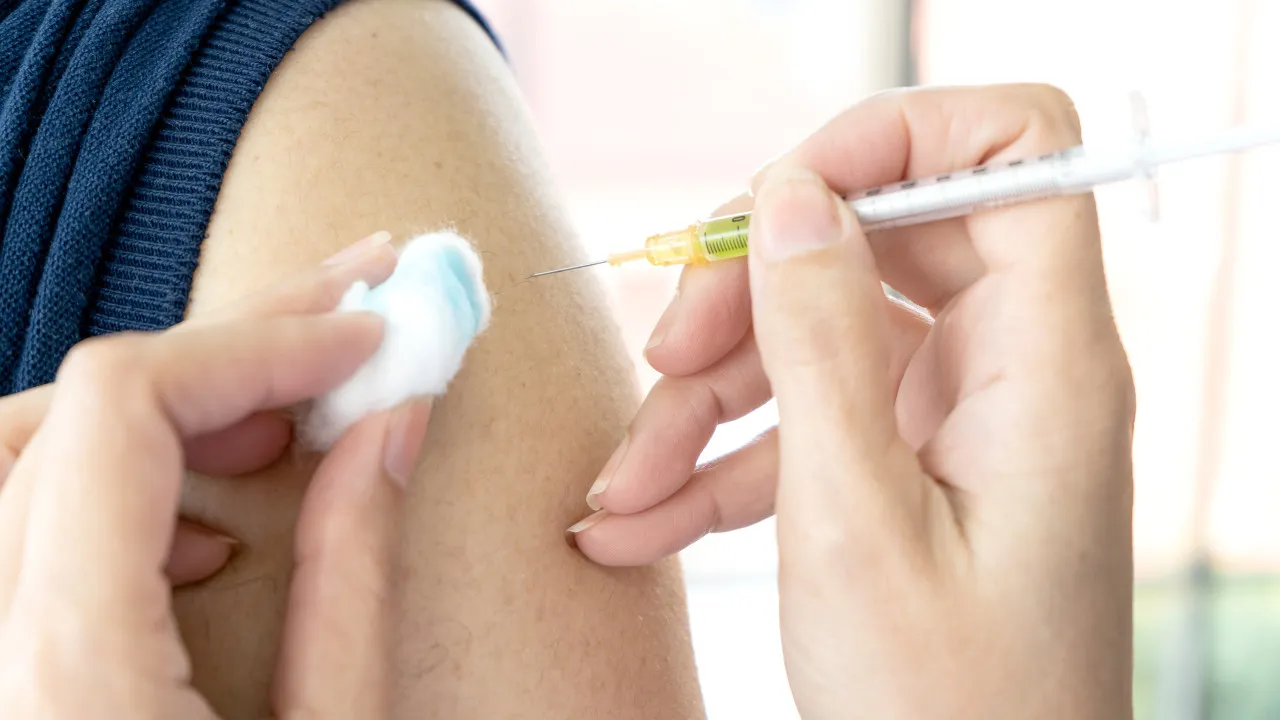
Zoonotic flu, a disease transferable between animals and humans, such as avian flu, can be prevented by a new vaccine. This directive, published Thursday, outlines access to the national strategic reserve of the medication.
The directive describes a strategy to vaccinate against zoonotic flu to mitigate the risk of a flu pandemic originating from zoonotic sources, focusing on pre-exposure to the virus and strengthening clinical and public health responses to human exposure cases.
Those eligible for the vaccine include members of rapid response teams managing zoonotic flu outbreaks in animals, laboratory technicians handling potentially contaminated samples, and employees of Wildlife Recovery Centers, the Nature and Environment Protection Service, nature guards of the Institute for Nature Conservation and Forests, and municipal veterinary services that directly interact with sick or dead birds.
The vaccination strategy aims to reduce the risk of zoonotic influenza virus transmission from infected animals to exposed individuals and the likelihood of human outbreaks.
Additionally, seasonal flu vaccination is recommended for individuals at increased risk of exposure to zoonotic influenza viruses to decrease the risk of coinfection by different genotypes and genetic rearrangement of the viruses.
In March, health authorities extended free flu vaccination to professionals at risk of direct exposure to sick or dead animals suspected of having zoonotic flu.
On January 7, the health authority published Guidance No. 001/2025 concerning “Zoonotic Flu or Other Influenza Virus of Animal Origin – Public Health and Clinical Approach,” addressing highly pathogenic avian flu outbreaks detected globally, including in Europe and Portugal.
This guidance for healthcare professionals covers early detection, case management, notification, diagnosis, treatment, epidemiological investigation, contact management, testing, chemoprophylaxis, vaccination, and risk communication based on the latest World Health Organization and European Centre for Disease Prevention and Control recommendations.
The health authority emphasizes that H5N1 transmission to humans is rare, with sporadic global cases. If infection occurs, severe clinical symptoms may manifest, typically within two to five days after the last exposure to sick or dead animals.
The disease primarily transmits in professional settings through direct or close contact with infected animals or their tissues, feathers, excrement, or inhalation of the virus from close contact with infected animals or contaminated environments.
To date, there is no evidence suggesting avian flu can be transmitted to humans through food consumption, including poultry meat and eggs.




