After the National Authority of Medicines and Health Products (Infarmed) suspended certain batches of the medication Sertralina Generis 50 mg and 100 mg, it also announced the suspension of some batches of Sertralina toLife 50 mg and 100 mg, film-coated tablets.
Infarmed reported that an “impurity above the acceptable limit” was detected.
According to an informational circular dated August 28, “Towa Pharmaceutical, S.A. will initiate a voluntary recall of the batches in question.”
As a result, “Infarmed mandates the immediate suspension of the commercialization of these batches.”
The authority advises that the entities with these batches in stock cannot sell, dispense, or administer them, and should proceed to return them.
Meanwhile, patients using medications from these batches should not stop treatment and should contact their doctor as soon as possible to obtain a different batch or an alternative medication.
The affected batches are listed as follows:
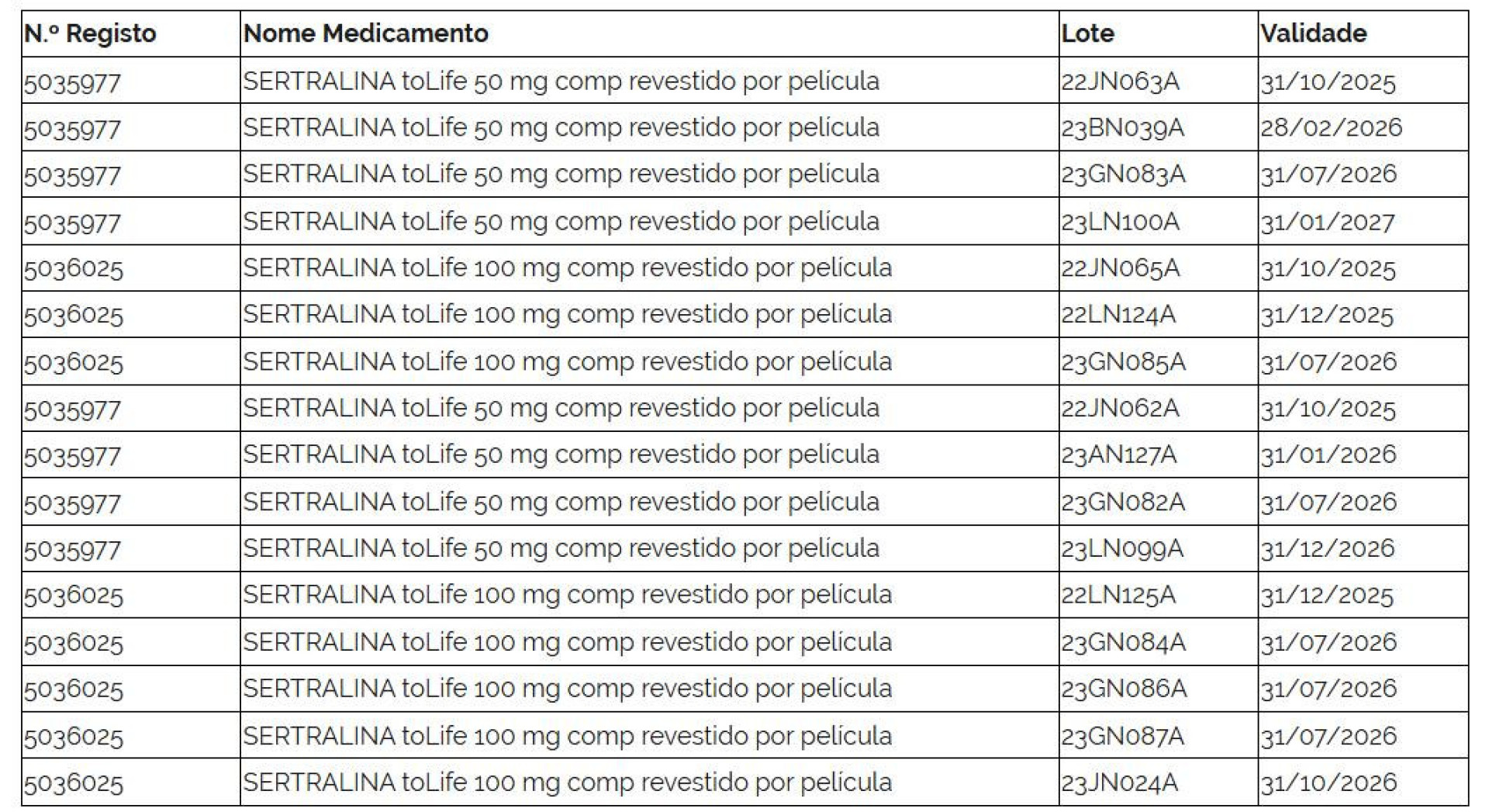
It is noteworthy that on August 28, Infarmed had already announced the suspension of the commercialization of certain batches of Sertralina Generis 50 mg and 100 mg.

Some batches of this medication, used to combat depression, were identified with abnormal amounts of an “impurity.”
Andrea Pinto | 12:42 – 29/08/2025




