The National Authority of Medication (Infarmed) has issued a warning regarding the “placement in the European market of devices from the manufacturer Dental Therapeutics AB” that bear the CE marking improperly.
“Infarmed became aware, through the competent authority of Sweden, of the placement of medical devices from the manufacturer Dental Therapeutics AB in the European market, displaying the CE marking improperly,” reads an informational circular published on their website.
It further states, “The devices in question are products intended for use in endodontic and exodontic procedures.”
Infarmed also mentions that “the certificate of conformity, issued by ON Interk Semko AB for these devices, was withdrawn on March 12, 2016,” and despite the withdrawal of the certificate, “the manufacturer continued to market the devices.”
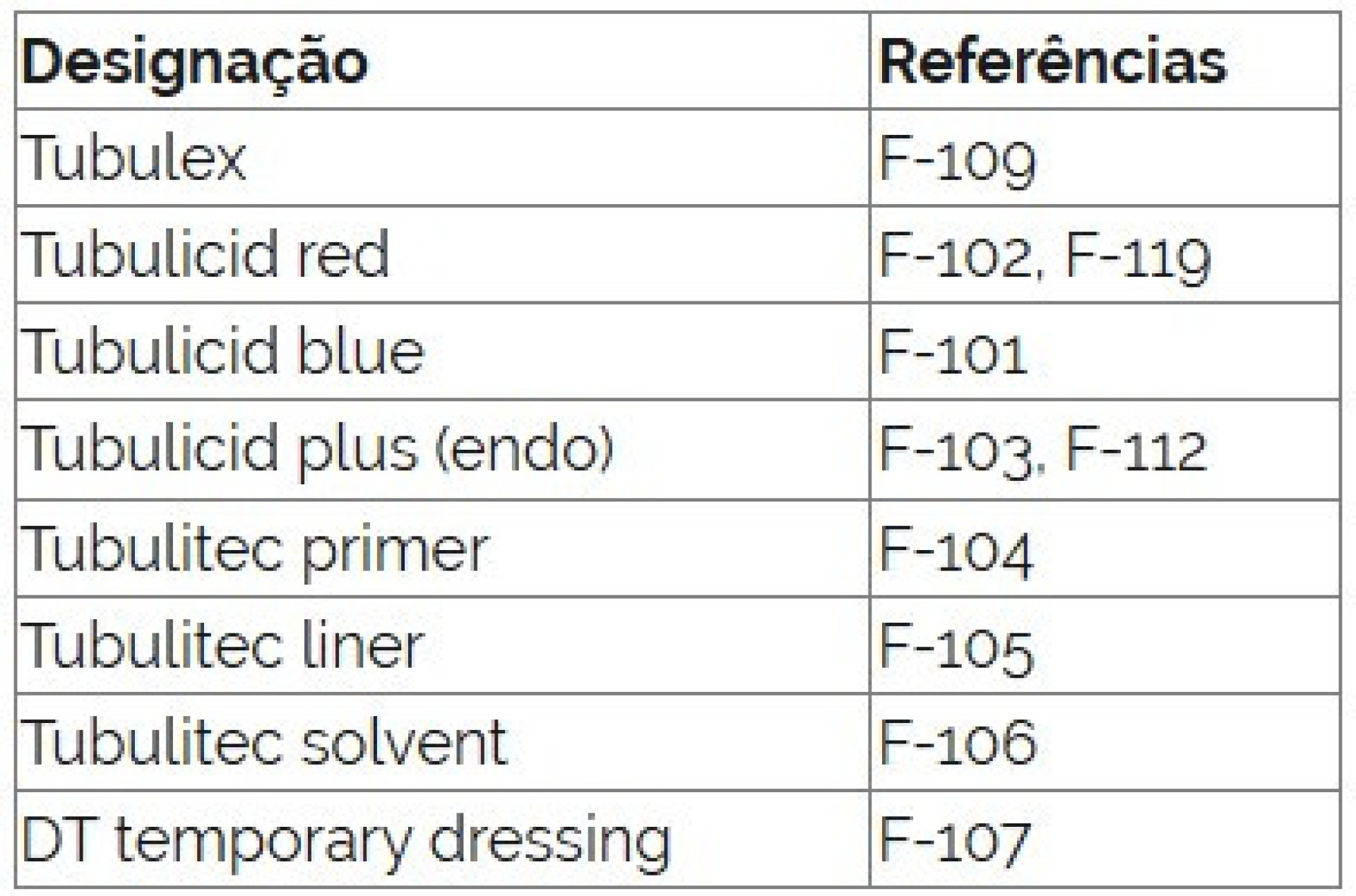
“It is further informed that the Swedish counterpart authority has ordered the prohibition of the placement and availability of the targeted medical devices in the market, as well as their withdrawal, with a view to restoring market compliance,” it reads.
The authority notes that, in Portugal, “to date, no records of devices from the manufacturer Dental Therapeutics AB have been identified.”
Nevertheless, “should these devices with a manufacturing date after March 12, 2016, and CE marking associated with code 0413 be detected in the national market,” Infarmed “recommends that these devices should neither be acquired nor used.”




